The NICE report of June 29th 2020 informed us that:
"There is no evidence to support taking vitamin D supplements to specifically prevent or treat COVID-19."
By this time there had been several important observational studies that demonstrated an association between low blood levels of vitamin D and increased risk of critical or fatal Covid-19. It had also been identified during previous decades that vitamin D has a pivotal role in activating the defensive immunity cascade, a role that has evolved during the past 500 million years. The early studies undertaken had measured blood levels of vitamin D in what were "snapshot" observations, but very valuable. Later studies demonstrated that a high risk of critical or fatal Covid-19 is predicted by low blood levels of vitamin D. Clinical research has been added to laboratory scientific research, and the two must always work together.
June 29th
NICE was not impressed by the research, but NICE has a very narrow view. Its role has been to evaluate new medicines to see if they are safe and effective to incorporate into the NHS. To achieve this a randomised controlled trial (RCT) is necessary, funded by the pharmaceutical company concerned.
But with vitamin D we are looking at something much more than a pharmaceutical product. We are concerned with a natural pre-hormone, cholecalciferol, that is produced in the body. It cannot be patented and no funding is automatically available for an RCT, which would always be very expensive if on large scale.
NICE is not orientated towards the evaluation of a very complex biological system such as defensive immunity, and this becomes clear on reading the NICE documents.
NICE is confused
In the statement "There is no evidence to support taking vitamin D supplements to specifically prevent or treat COVID-19" we see a good example of cryptic double-speak. Taking vitamin D supplements was not part of the evidence available to NICE at the time. The NICE comment was not appropriate. It also failed to acknowledge that a treatment for an infection or other disease cannot be undertaken in advance of its emergence.
An evaluation of "supplements" would have involved an RCT which had not at the time been undertaken. To set up such an RCT takes considerable time. A primary prevention trial involving normal people would necessitate very large numbers at prohibitively high cost to cover logistics, staffing, follow-up etc.This is beyond the scope even of vaccine evaluation. An RCT of patients with definite and serious Covid-19 infection would involve many fewer subjects and could be performed in any acute hospital.
Ethical approval
For a clinical trial, ethical approval is essential, with informed consent being given by the subjects. With existing knowledge concerning the benefits of vitamin D in Covid-19, the main issue would be whether it would be ethical to withhold vitamin D from the control group of patients. Human sacrifice should not be part of clinical research.
The main development of the RCT followed the serious side-effects produced by the drug thalidomide when given to expectant mothers. It is safety of a medicine that is arguably more important than its effectiveness, and this is to be assessed by the RCT. However, with vitamin D we know that it is very safe, with reversible overdose effects being the result of a rare disease (eg sarcoidosis) or a serious dosing error, such as mistaking milligrams for micrograms and giving 1000 times the intended dose. 100mcg or 100µg is 4,000 units, but 100mg is 4,000,000 units and taking this would be a serious error. It is much safer to keep to international units rather than potentially confusing mass units.
NICE is single-minded. RCT is its currency. It would not be able to evaluate, for example, the relationship between cigarette smoking and lung cancer, and this would be outside its remit. Similarly it was not able to evaluate the relationship between vitamin D and Covid-19. The government had no other agency to turn to, and its Chief Scientist Sir Patrick Vallance and Chief Medical Officer Professor Chris Whitty remained silent on the subject. Silence was similarly maintained by the Royal College of Physicians, the traditional source of medical wisdom during the past 500 years.
We are dealing with a pre-hormone deficiency
NICE was answering the wrong question. The question or challenge posed to NICE in June 2020 was not concerned with vitamin D supplements. It was concerned with the observations that vitamin D deficiency is associated with an unfavourable and perhaps fatal outcome from Covid-19 infection. NICE should have declared itself not able to comment on this challenge, not able to evaluate the immunological disadvantages of vitamin D deficiency.
But the government and its advisors took the NICE report to indicate that vitamin D itself was of no value in the Covid-19 pandemic and so no action was taken.
Other examples
Much observational evidence was presented, but NICE was not tempted to comment. Its lack of vision means that, had it been established at the time, NICE would have rejected the careful observational data that led to the conclusion that cigarette smoking causes lung cancer. Consider setting up a double blind RCT of cigarette smoking. Similarly the relationship between alcohol consumption by car drivers and subsequent road traffic accidents would create a challenge to those who would insist on an RCT.
There are other examples of hormone deficiency states. One is thyroid under-activity, which can be corrected easily by the use of thyroid hormone (thyroxine) supplements. This has never been subjected to an RCT, but by good fortune NICE had not been established when thyroid replacement therapy was introduced. Similarly Type 1 diabetes is a life-threatening deficiency of insulin, but insulin had never been subjected to an RCT. Was one really necessary? Also, cortisol replacement in Addison's disease was not subjected to an RCT.
The absurdity of demanding an RCT for correction of vitamin D deficiency is obvious.
Safety
The most interesting example is that the use of a parachute when gravitationally challenged by jumping out of an airborne aircraft has never been subjected to an RCT. Nevertheless it has been accepted throughout the world as the sensible thing to do, as observation indicates that injuries are far fewer in those who use parachutes compared o those who (inadvertently) don't. NICE members might volunteer for an RCT should it be demanded.
It is obvious to anyone with sense that observational studies are very valuable and RCTs have very limited use in the real world, especially when evaluating safety. It is safety that is the objective when correcting vitamin D deficiency. We are not dealing with a pharmaceutical agent.
NICE stated that it would not approve the use of vitamin D unless it was supported by a positive RCT. It was predictable that this might occur and cause some discomfort to NICE in forthcoming months. "No evidence" might be found to be a serious error.
RCT from Córdoba
September 3rd 2020 saw the results of an RCT from Córdoba, Spain. This has been reviewed earlier. To summarise, 26 randomly allocated patients with Covid-19 pneumonia received standard care, and 50 received standard care plus Calcifediol.
Calcifidiol is 25(OH)Vitamin D, a part activated form. It is produced naturally from vitamin D (derived from the skin or from the diet) on circulation through the liver. When we test blood vitamin D level it is 25(OH)D that is measured. It appears that the blood level of 25(OH)D increases much more quickly when Calcifidiol is given rather than vitamin D. This would be a great advantage in the seriously ill.
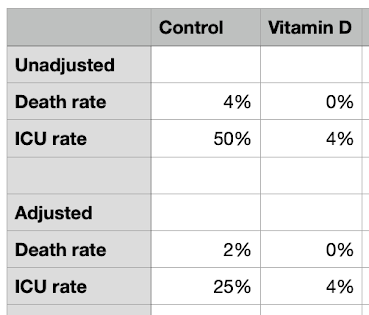
In the control group of 26, 13 (50%) required ICU support and 2 died. In the group receiving vitamin D as Calcifediol only 1 (2%) required ICU and there were no deaths.
This result shows a dramatic benefit from vitamin D as Calcifediol. Putting this together with the large amount of existing evidence (15 studies) and underlying laboratory science, as an optimist and pragmatist I anticipated that it would be incorporated into clinical practice. The basis of this would be that vitamin D is readily available, cheap, and known to be very safe. There would be the opportunity to reduce critical and fatal Covid-19 with no downside.
The choice would be as described by Pascal's wager.
But no official change took place. The NICE proclamation of June 29th was set in stone.
NICE Report September 2020
NICE had demanded an RCT of vitamin D, and here it was. NICE had to be stirred into action to review its position with new knowledge from Córdoba, Spain, and in response it published a new interim report at the end of September.
The title is:
Vitamin D supplementation for preventing intensive care admissions in people with COVID-19 associated pneumonia.
It set out to assess the paper from Córdoba, by Castillo et al. "Assess" meant destroy.
It started: [The study] "found that vitamin D supplementation in hospitalised adults with COVID-19 may reduce admission to intensive care".
The word "may" is not appropriate: it demonstrated "does" reduce admission to intensive care.
"However, the study has many confounders so the results should be interpreted with caution."
"The clinical management of patients with COVID-19 should not be changed based on the results of this study."
The traditional method of clinical practice in the UK and elsewhere is that physicians would read original papers, discuss them with colleagues, assimilate them with previous knowledge, and then decide whether or not to incorporate them into clinical practice. This is no longer what happens: thinking and clinical judgement are no longer acceptable. What is written by NICE is effectively the law, as departures from NICE guidelines cannot be legally defended.
NICE stated that clinical practice should not be changed and at least officially it wasn't changed. But individual doctors might have broken the rules and given vitamin D or Calcifediol to help patients at risk. Was there any reason to deny a likely life-saving treatment from patients with serious Covid-19, apart from the dogma of NICE?
How to rubbish a good trial: a lesson from NICE
The only way to stop vitamin D was to show that the RCT from Córdoba was of no value and could not be trusted to convey the truth. The attack was based on "confounders". This can always be done.
When an RCT is undertaken, the subjects entering the study are randomly allocated to either the control group or the special treatment group. Random allocation is to make certain that the two groups are as similar as possible in respect of factors that might influence the results. One would be age, and if the average age of one group was significantly different from the other group this might influence the result and cast doubt upon its truth. This is a confounder.
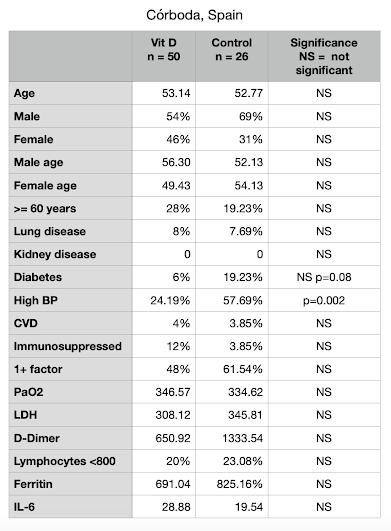
The RCT from Córdoba presented all the potential confounders and they can be seen in the table.
It is then necessary to check differences for statical significance, this usually being summarised by a "p value". The smaller the p, the more likely is the significance of difference. NS means not significant.
Ideally the differences of the characteristics in the two groups should show almost complete overlap on the distribution curves, indicating no significant difference, as illustrated below left. But there might be a separation, indicting a significant difference.
The ideal is as in the first pair with means (averages) 14 and 18. Perfect matching cannot be expected and we can see this in the two columns above. But the second pair shows a degree of separation, with means 14 and 25. This not likely to be significant, but when the separation becomes greater, the "confounding" becomes greater. The p-value can be calculated from the caparisons, adjusting the means for the sample size and the spread of the data (variance), comparing two "standard errors" and reading off the p-value from a standard table.In the Córdoba RCT, only one confounding variable showed a statistically significant difference, and this was "a previous history of high blood pressure". 21.49% of the vitamin D treated groups had a history of high blood pressure, compared to 57.69% in the control group. This difference was statistically significant, with the control group put at a greater risk of serious illness than the vitamin D treated group. The question is, would this difference be so important that the study would be invalidated?
NICE decided that the results of the trial were invalid on the basis of an excess of subject with high blood pressure in the control group. We know that such people are at greater than average risk when experiencing Covid-19 infection, and this can be quantified. It is thought that people with previous high blood pressure have twice the risk of death as those without.
Let us adjust for this by dividing by two the unfavourable outcomes for the control group.
Let us ignore the death rates as the numbers are so small (1 versus 0),
We can see however that the adjusted ICU admission rate is 25% in the control group and 4% in the vitamin D treated group. This remains a very significant difference.
Correction in this way for a confounder is perfectly in order and it probably over-corrects.
Another criticism from NICE
The NICE report offered another criticism, that the paper did not make it clear if the ward doctors knew which patients had been given vitamin D on admission. If they did know, they might have kept the Calcifediol treated group away from intensive care, but that might have increased deaths. Ideally those not receiving Calcifediol should have been given a placebo, so as to "blind" the attending doctors.
I quote from the Córdoba paper:
An electronically generated randomization 2:1 list was prepared by independent statisticians. The list was accessible only to nonmasked specialists in the study in an attempt to minimize observation bias. The patients' data were recorded in the hospital's electronic medical record, with blind access by the technical data collectors and the statistician who carried out the study.
Clinical research is very difficult to control and the researchers must do their best to avoid observation bias, as in this study. In retrospect it would have been better to give the control patients a dummy capsule, a placebo to minimise observation bias further.
Should this criticism invalidate the results of the trial? I think not.
The weight of evidence
The Córdoba study was not in isolation but it should be taken into consideration with other studies the results of which were available but which NICE chose to ignore.
NICE proposed to delay a decision on the value of a vitamin D supplement until more RCT results become available. When will this be? To reproduce the Córdoba trial would be simple, in a one or group of several UK hospitals.
I am aware that the Chief Medical Officer Professor Chris Whitty has refused at least one major hospital permission to undertake such a study. Furthermore, The Bill & Melinda Gates Foundation / Wellcome Foundation consortium the Covid-19 Accelerator has refused funding for vitamin D related research.
What is happening to our long tradition of good quality clinical research?
But what are the staff in the UK hospitals to do in the meantime? Acting on the basis of the weight of evidence available at the time, and knowing that vitamin D at the doses given is perfectly safe, they should have accepted Pascal's wager and started to treat patients with Covid-19 pneumonia on the Córdoba protocol. The least they could have done would be to give patients with Covid-19 pneumonia the option of receiving vitamin D as Calcifediol, informing them of the possible dangers (zero) and the possible benefits, 25% rather than 2% risk of needing transfer to ICU.
Edict from NICE
Edict = an official order or proclamation issued by a person in authority.
As we seen, any initiative taken by doctors was forbidden by NICE. To repeat:
"The clinical management of patients with COVID-19 should not be changed based on the results of this study."
This is staggering centralisation of power over the medical practitioners of the land, denying them the use of clinical judgement, and more importantly denying the patients a say concerning their treatment. "NICE knows best" is ultimate paternalism.
The doctors and other staff would want to do their best for their patients. But they have had to deny their patients a treatment that is known to be perfectly safe and which might be life-saving. Furthermore, the patients and their families were not given the opportunities to decide for themselves whether or not to take Calcifediol / Vitamin D, given the information that would be easily assimilated.
NICE sits in a metaphorical ivory tower away from the sharp end of medical practice. There will never be blood on the floor of the NICE offices.
But since the Córdoba results appeared on September 3rd until October 22nd there have been 2,803 deaths in the UK from Covid-19. This is a scandal and the occurrence of many of these deaths must be the responsibility of the myopia and intransigence of NICE.
 |
| This is NOT the floor of the NICE office |